My cathodic ED bath has low pH?
Product Support and Customer Service
For Further support visit our Contact Page
Safety
Think and act in a safe manner. Always disconnect power and use a lockout before you work on the E-coat system, or any of the related subsystems. Observe any confined space conditions. Use the appropriate safety equipment and clothing for the task. Please carefully read all the instructions listed below to familiarize yourself with the project before attempting to perform any of the work.
Required Materials
- Titration Equipment, Titration Solutions
Required Tools
- None
General
Generally, the Membrane Electrode System will remove more neutralizer than necessary and thus cause the cathodic ED bath pH to move to the high side. So a condition where the ED bath pH is low is unusual. The cathodic ED bath loses neutralizer (acid) in one of four ways: UF permeate purges; dragout of rinse from the post rinses; small amounts of acid in the e-coat film; and finally by the Membrane Electrode System. Generally, the first three losses are somewhat fixed for by such factors as production rate, ware geometry, ware mix, local plant practice, etc. The Membrane Electrode system will remove neutralizer based upon the number of Coulombs (I.E. amp * seconds), however the net removal rate is always less because some of the neutralizer is able to rejoin the ED bath. Acid is added to the ED bath through new ED replenishment paint and direct acid additions. Acid also returns back to the ED bath as it cascades from the post rinses. If the net neutralizer removal rate is decreased then the operator of the ED system will begin to see a reduction in the pH of the ED bath. The obvious question to ask is what happened to cause a reduction of the net neutralizer removal rate? New ED systems that start up slowly are also prone to low ED bath pH, but not generally from the reasons shown below. There are several conditions that are probable –
- Increase in the back diffusion of neutralizer from the Membrane Electrode System into the ED bath.
- Leak of electrolyte back into the ED bath.
- Decrease in the transmission of anions across the membrane of the Membrane Electrode Cells (i.e. decrease in the basic ion removal rate).
- Increased carry over of low pH material from the pre-treatment system.
- Excessive amount of direct acid additions
- Malfunctioning pH meter
- More neutralizer (I.E. higher MEQ acid) in the ED replenishment paint.
The purpose of this Service Reference is to discuss each of the items that can result in a low bath pH. For each condition, some sort of investigation is called for and it will also detail these appropriate steps.
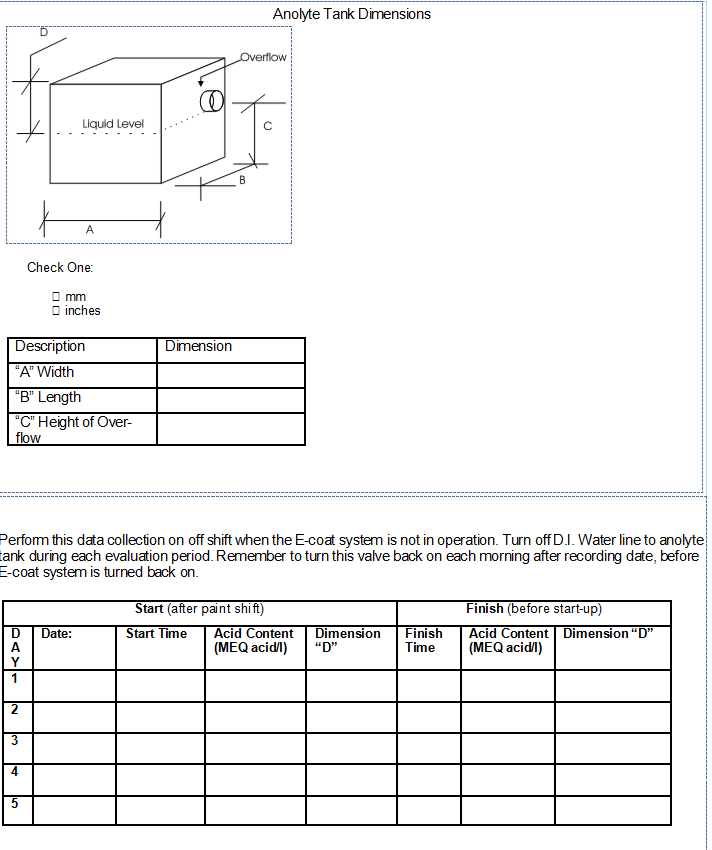
Increase in the back diffusion of neutralizer from the Membrane Electrode System to the ED bath. Back diffusion is the process by which a difference in the concentration across a semi-permeable membrane is equalized. Some would refer to this as osmosis. Generally, the concentration of neutralizer is several times greater than what is in the ED bath. The back diffusion rate is the greatest during periods then where is no cathode in the ED bath. When the ED process is on-going the voltage gradient and resulting polarization driving force (towards the Cell) around the Cell is much, much greater than the osmotic pressure driving force (outwards from the Cell). Back diffusion is always happening and trying to understand why it has increased can be a challenge. How often have the Membrane Shells been allowed to completely dry out? Have any Membrane Shell been hit or bruised recently? You may follow the procedure below to perform an analytical check to estimate the amount of back diffusion that your system has and this figure can be compared to other typical systems. Anolyte Tank Dimensions
Perform this data collection on off shift when the ED system is not in operation. Turn off D.I. Water line to anolyte tank during each evaluation period. Remember to turn this valve back on each morning after recording date, before e-coat system is turned back on. Use the attached form to take readings for five (5) consecutive off periods. It is necessary to measure the acid concentration and report the figures in MEQ acid/liter. Fax this chart back to UFS for analysis and a report.
Leak of electrolyte back into the ED bath.
If there is a leak of electrolyte (I.E. anolyte) back into the ED bath, then the net removal rate of neutralizer is greatly reduced and this will tend to suppress the ED bath pH. This is most often seen first in a lower level of electrolyte in the electrolyte circulation tank. See Bulletin #990103 for complete details on this procedure.
Decrease in the transmission rate of anions across the membrane of the Membrane Electrode Cells.
As the membrane ages or undergoes contamination by fouling agents, it ability to attract anions is decreased as well. For most systems, the ED operators will also see an increase in the voltage set point too. In order to analyze this, it is necessary to shut off the DI input to the Electrolyte Circulation System and then take a ‘before’ and ‘after’ measurement of the MEQ acid in the electrolyte. If the number of Coulombs is known (I.E. during the period of the test), then the transmission rate can be estimated and then compared with other systems. Perform this data collection on when the ED system is in operation. Turn off D.I. Water make-up line to Electrolyte tank during each evaluation period. Remember to turn this valve back on each morning after the test is concluded for that day. Use the form below to take readings for at least 3 evaluation periods. It is necessary to measure the acid concentration and report the figures in MEQ acid/liter. For monorail type ED systems use the steady state current reading, if the load changes and the current increases or decreases, then the average current value should be adjusted too. For hoist type ED Systems, the average current is more difficult to determine since the current falls off as the ED film is developing. If you can graph current verses time, then Coulombs is the integral under that curve. Contact UFS if you for more assistance.
| Day | Start Time | Start MEQ Acid (MEQ/liter) | Finish Time | Finish MEQ Acid (MEQ/liter) | Average Current (amps) |
| 1 | |||||
| 2 | |||||
| 3 | |||||
| 4 | |||||
| 5 |
Fax this chart back to UFS for analysis and a report.
Increased carry over of low pH material from the pre-treatment system.
This not very likely since the last stage before the ED tank is usually a DI water rinse. Take a sample of the last pre-treatment stage rinse and analyze. Perform the same test on the dripping being carried over into the ED bath.
Excessive amount of direct acid additions
This also is not likely, but is a possible contributor Review the operator’s log sheet for the amount and type of acid added to the ED bath. Review the acid addition techniques & methods, if necessary.
Malfunctioning pH meter
Confirm that the pH meter is properly calibrated and is functioning properly. Perform the calibration of the pH meter as described by the manufacturer, use fresh pH standard solutions, and make sure the pH probe is properly conditioned and filled with the appropriate electrolyte.
More neutralizer (I.E. higher MEQ acid) in the ED replenishment paint.
Is there more neutralizer in the replenishment ED paint? Ask your ED paint supplier for guidance on this question.
BULLETIN 990144